01
J Clin Invest. 20230223
✦
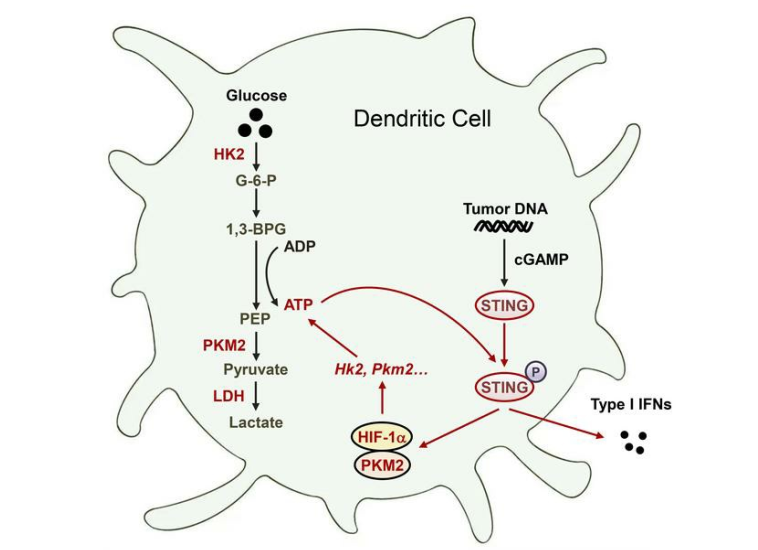
Glycolysis drives STING signaling to facilitate dendritic cell antitumor function
糖酵解通过驱动STING信号通路促进树突状细胞的抗肿瘤功能
J Clin Invest. 2023 Feb 23;e166031.
doi: 10.1172/JCI166031.
PubMed: https://pubmed.ncbi.nlm.nih.gov/36821379/
Abstract
Activation of STING signaling in dendritic cells (DCs) promotes antitumor immunity. Aerobic glycolysis is a metabolic hallmark of activated DCs, but how the glycolytic pathway intersects with STING signaling in tumor-infiltrating DCs remains elusive. Here, we show that glycolysis drives STING signaling to facilitate DC-mediated antitumor immune responses. Tumor-infiltrating DCs exhibited elevated glycolysis, and blockade of glycolysis by DC-specific Ldha/Ldhb double deletion resulted in defective antitumor immunity. Mechanistically, glycolysis augmented ATP production to boost STING activation and STING-dependent DC antitumor functions. Moreover, DC-intrinsic STING activation accelerated HIF-1a-mediated glycolysis and established a positive feedback loop. Importantly, glycolysis facilitated STING-dependent DC activity in tissue samples from non-small cell lung cancer patients. Our results provide mechanistic insight into how the crosstalk of glycolytic metabolism and STING signaling enhances DC antitumor activity and can be harnessed to improve cancer therapies.
树突状细胞(DCs)中STING信号的激活可促进抗肿瘤免疫。有氧糖酵解是活化DC的代谢标志,但在肿瘤浸润性DC中,糖酵解途径如何与STING信号产生交互仍不清楚。在本研究中,我们发现糖酵解通过驱动STING信号通路促进了DC介导的抗肿瘤免疫应答。肿瘤浸润性树突状细胞表现出糖酵解水平的升高,而通过DC特异性的Ldha/Ldhb双敲除来阻断糖酵解会导致抗肿瘤免疫的缺陷。在机制上,糖酵解增加ATP的产生,从而促进了STING的激活和依赖STING的DC抗肿瘤功能。此外,DC固有的STING激活加速了HIF-1a介导的糖酵解并形成了正反馈环路。重要的是,糖酵解促进了非小细胞肺癌患者组织样本中STING依赖的DC活性。我们的研究结果提供了关于糖酵解代谢和STING信号如何相互作用增强DC抗肿瘤活性的机制,并有望用于改善癌症治疗。
Keywords:
Cancer immunotherapy; Glucose metabolism; Immunology; Innate immunity; Metabolism.
癌症免疫治疗;葡萄糖代谢;免疫学;先天免疫;代谢。
02
Mol Cancer. 20230216
✦
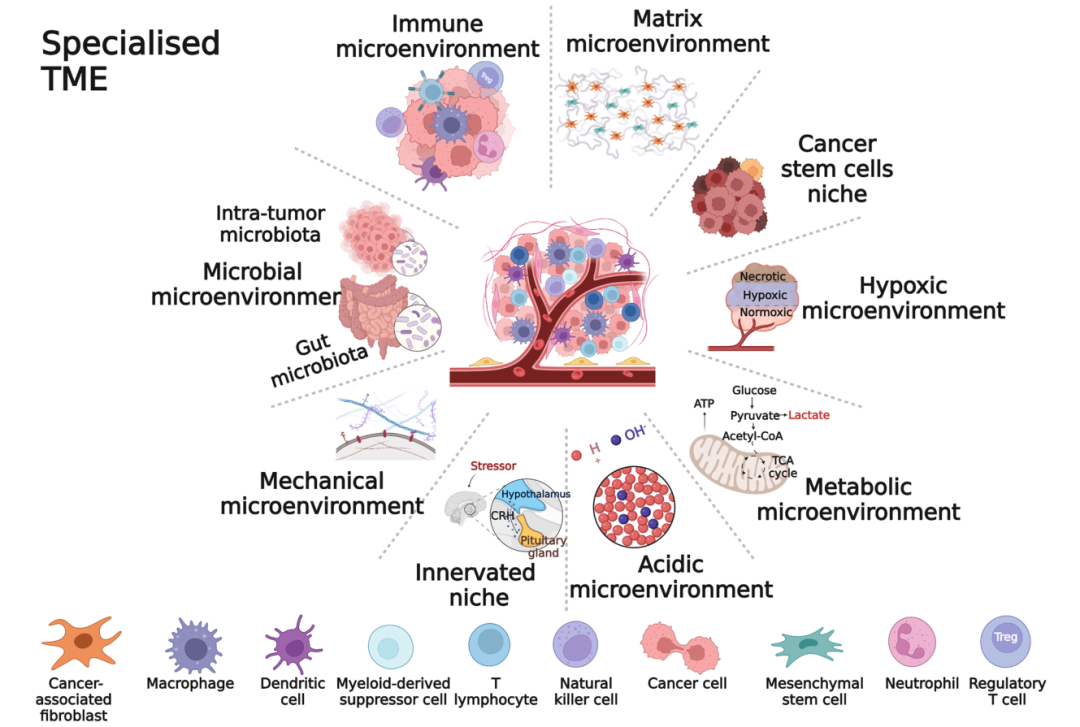
NK cells are never alone: crosstalk and communication in tumour microenvironments
NK细胞与肿瘤微环境间交流和相互作用
Mol Cancer. 2023 Feb 16;22(1):34.
doi: 10.1186/s12943-023-01737-7.
PubMed: https://pubmed.ncbi.nlm.nih.gov/36797782/
Abstract
Immune escape is a hallmark of cancer. The dynamic and heterogeneous tumour microenvironment (TME) causes insufficient infiltration and poor efficacy of natural killer (NK) cell-based immunotherapy, which becomes a key factor triggering tumour progression. Understanding the crosstalk between NK cells and the TME provides new insights for optimising NK cell-based immunotherapy. Here, we present new advances in direct or indirect crosstalk between NK cells and 9 specialised TMEs, including immune, metabolic, innervated niche, mechanical, and microbial microenvironments, summarise TME-mediated mechanisms of NK cell function inhibition, and highlight potential targeted therapies for NK-TME crosstalk. Importantly, we discuss novel strategies to overcome the inhibitory TME and provide an attractive outlook for the future.
免疫逃逸是癌症的标志。动态的异质性肿瘤微环境(TME)导致自然杀伤(NK)细胞免疫治疗的浸润不足和疗效不佳,这成为触发肿瘤进展的关键因素。了解NK细胞和TME之间的相互作用为优化基于NK细胞的免疫治疗提供了新的见解。本文介绍了NK细胞与免疫、代谢和微生物微环境等9种特定TME之间直接或间接交互作用的新进展,总结了TME介导的NK细胞功能抑制机制,并提出了针对NK细胞与TME交互作用的潜在靶向治疗方法。重要的是,我们讨论了克服抑制性TME的新策略,并对未来提出了展望。
Keywords:
Cancer; Communication; Crosstalk; NK cells; TME.
癌症;交流;交互;NK细胞;肿瘤微环境。
03
Nat Rev Cancer. 20230215
✦
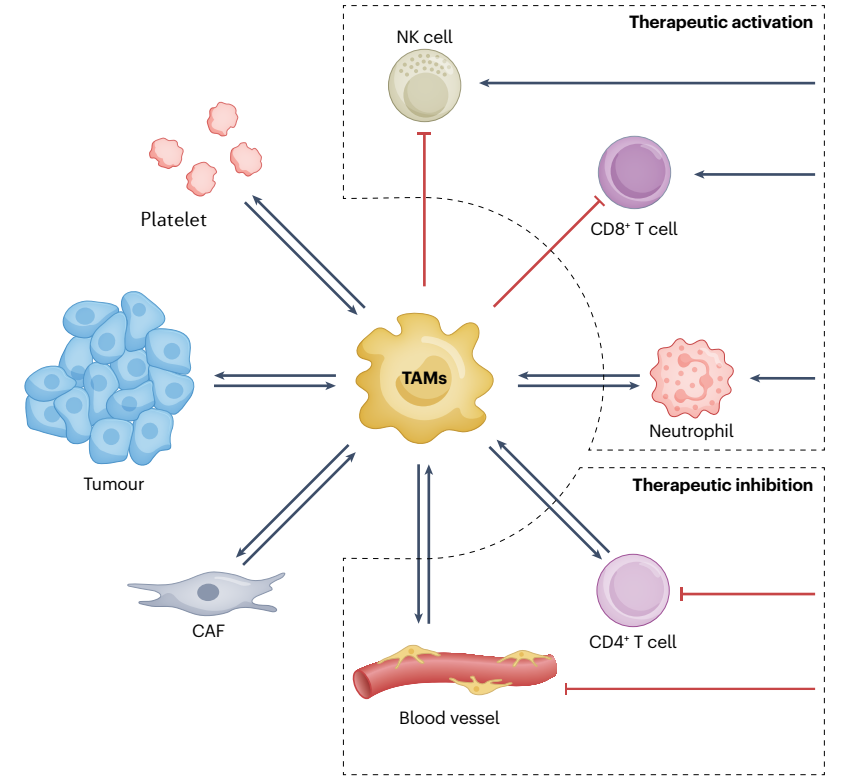
A timeline of tumour-associated macrophage biology
肿瘤相关巨噬细胞的生物学特征
Nat Rev Cancer. 2023 Feb 15.
doi: 10.1038/s41568-022-00547-1.
PubMed: https://pubmed.ncbi.nlm.nih.gov/36792751/
Abstract
Tumour progression is modulated by the local microenvironment. This environment is populated by many immune cells, of which macrophages are among the most abundant. Clinical correlative data and a plethora of preclinical studies in mouse models of cancers have shown that tumour-associated macrophages (TAMs) play a cancer-promoting role. Within the primary tumour, TAMs promote tumour cell invasion and intravasation and tumour stem cell viability and induce angiogenesis. At the metastatic site, metastasis-associated macrophages promote extravasation, tumour cell survival and persistent growth, as well as maintain tumour cell dormancy in some contexts. In both the primary and metastatic sites, TAMs are suppressive to the activities of cytotoxic T and natural killer cells that have the potential to eradicate tumours. Such activities suggest that TAMs will be a major target for therapeutic intervention. In this Perspective article, we chronologically explore the evolution of our understanding of TAM biology put into the context of major enabling advances in macrophage biology.
肿瘤的进展受局部微环境的调节。这个环境中有许多免疫细胞,其中巨噬细胞是最丰富的。临床相关数据和大量小鼠肿瘤模型的临床前研究表明,肿瘤相关巨噬细胞(TAMs)具有促癌作用。在原发肿瘤中,TAMs促进肿瘤细胞侵袭和内渗,促进肿瘤干细胞活性并诱导血管生成。在转移部位,与转移相关的巨噬细胞促进外渗、肿瘤细胞存活和持续生长,并在某些情况下维持肿瘤细胞休眠。在原发灶和转移灶中,TAMs均可抑制细胞毒性T细胞和自然杀伤细胞的活性,而这些细胞具有消灭肿瘤的潜力。这些证据提示TAMs将成为治疗干预的主要靶点。在这篇文章中,我们将巨噬细胞生物学的主要进展按时间顺序进行归纳探讨,以加深我们对TAM生物学的理解。
04
Mol Cancer. 20230210
✦
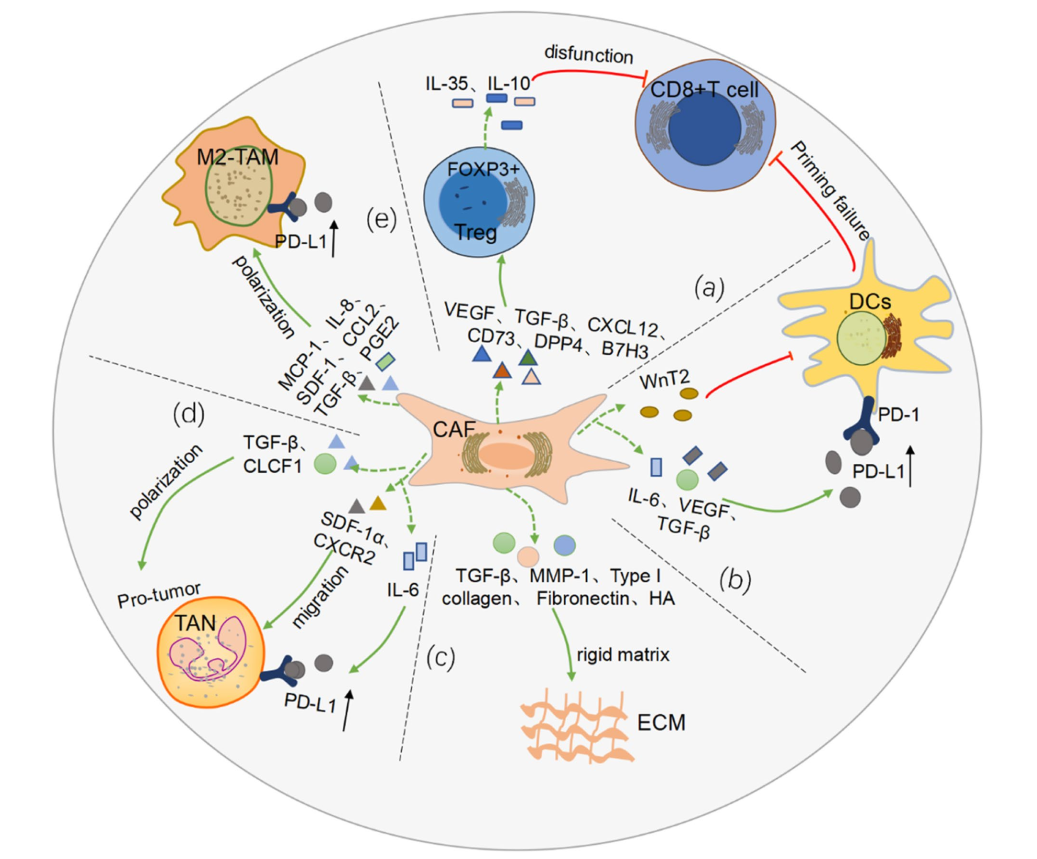
Roles of cancer-associated fibroblasts (CAFs) in anti- PD-1/PD-L1 immunotherapy for solid cancers
肿瘤相关成纤维细胞(CAFs)在抗PD-1/PD-L1实体癌免疫治疗中的作用
Mol Cancer. 2023 Feb 10;22(1):29.
doi: 10.1186/s12943-023-01731-z.
PubMed: https://pubmed.ncbi.nlm.nih.gov/36759842/
Abstract
In recent years, breakthroughs have been made in tumor immunotherapy. However, tumor immunotherapy, particularly anti-PD-1/PD-L1 immune checkpoint inhibitors, is effective in only a small percentage of patients in solid cancer. How to improve the efficiency of cancer immunotherapy is an urgent problem to be solved. As we all know, the state of the tumor microenvironment (TME) is an essential factor affecting the effectiveness of tumor immunotherapy, and the cancer-associated fibroblasts (CAFs) in TME have attracted much attention in recent years. As one of the main components of TME, CAFs interact with cancer cells and immune cells by secreting cytokines and vesicles, participating in ECM remodeling, and finally affecting the immune response process. With the in-depth study of CAFs heterogeneity, new strategies are provided for finding targets of combination immunotherapy and predicting immune efficacy. In this review, we focus on the role of CAFs in the solid cancer immune microenvironment, and then further elaborate on the potential mechanisms and pathways of CAFs influencing anti-PD-1/PD-L1 immunotherapy. In addition, we summarize the potential clinical application value of CAFs-related targets and markers in solid cancers.
近年来,肿瘤免疫治疗取得了突破性进展。然而,肿瘤免疫疗法,尤其是抗PD-1/PD-L1免疫检查点抑制剂,仅对一小部分实体癌患者有效。如何提高肿瘤免疫治疗的效率是一个亟待解决的问题。众所周知,肿瘤微环境(tumor microenvironment, TME)状态是影响肿瘤免疫治疗有效性的重要因素,其中肿瘤相关成纤维细胞(cancer-associated fibroblasts, CAFs)近年来备受关注。CAFs作为TME的主要成分之一,通过分泌细胞因子和囊泡与癌细胞以及免疫细胞相互作用,参与ECM重塑,最终影响免疫应答过程。随着对CAFs异质性研究的深入,为寻找联合免疫治疗靶点和预测免疫疗效提供了新的策略。本文将围绕CAFs在实体肿瘤免疫微环境中的作用,进一步阐述CAFs影响抗PD-1/PD-L1免疫治疗的潜在机制和通路。此外,本文还总结了CAFs相关靶点和标志物在实体癌中的潜在临床应用价值。
Keywords:
Cancer-associated fibroblasts; Immunotherapy; PD-1/PD-L1 inhibitors.
肿瘤相关成纤维细胞;免疫治疗;PD-1/PD-L1抑制剂。
05
Cancer Cell. 20230209
✦
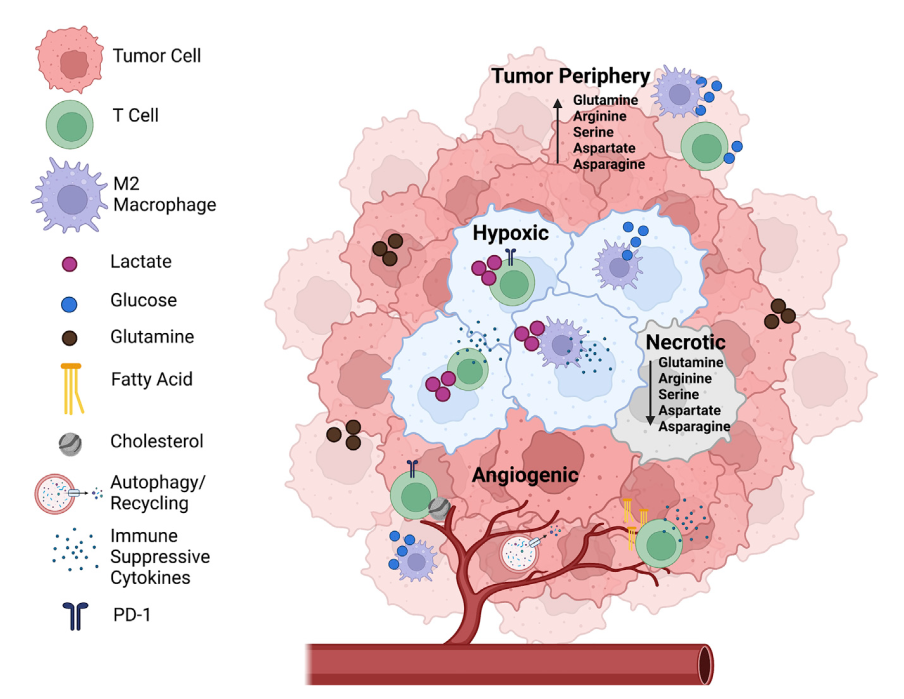
Metabolic programming and immune suppression in the tumor microenvironment
肿瘤微环境中的代谢编程和免疫抑制
Cancer Cell. 2023 Feb 9;S1535-6108(23)00009-0.
doi: 10.1016/j.ccell.2023.01.009.
PubMed: https://pubmed.ncbi.nlm.nih.gov/36801000/
Abstract
Increased glucose metabolism and uptake are characteristic of many tumors and used clinically to diagnose and monitor cancer progression. In addition to cancer cells, the tumor microenvironment (TME) encompasses a wide range of stromal, innate, and adaptive immune cells. Cooperation and competition between these cell populations supports tumor proliferation, progression, metastasis, and immune evasion. Cellular heterogeneity leads to metabolic heterogeneity because metabolic programs within the tumor are dependent not only on the TME cellular composition but also on cell states, location, and nutrient availability. In addition to driving metabolic plasticity of cancer cells, altered nutrients and signals in the TME can lead to metabolic immune suppression of effector cells and promote regulatory immune cells. Here we discuss how metabolic programming of cells within the TME promotes tumor proliferation, progression, and metastasis. We also discuss how targeting metabolic heterogeneity may offer therapeutic opportunities to overcome immune suppression and augment immunotherapies.
葡萄糖代谢和摄取增加是许多肿瘤的特征,临床上用于诊断和监测癌症进展。除癌细胞外,肿瘤微环境(tumor microenvironment, TME)还包括广泛的基质、固有和适应性免疫细胞。这些细胞群之间的合作和竞争支持肿瘤的增殖、进展、转移和免疫逃逸。细胞异质性导致代谢异质性,因为肿瘤内的代谢程序不仅取决于TME细胞的组成,还取决于细胞的状态、位置和养分的可利用性。除了驱动癌细胞的代谢可塑性,TME中营养物质和信号的改变可导致效应细胞的代谢性免疫抑制,促进调节性免疫细胞。本文将讨论TME中细胞的代谢编程如何促进肿瘤的增殖、进展和转移。我们还讨论了靶向代谢异质性如何为克服免疫抑制和增强免疫治疗提供治疗机会。
Keywords:
immune; metabolism; metastasis; plasticity; tumor microenvironment.
免疫;代谢;转移;可塑性;肿瘤微环境。
06
Cancer Cell. 20230209
✦
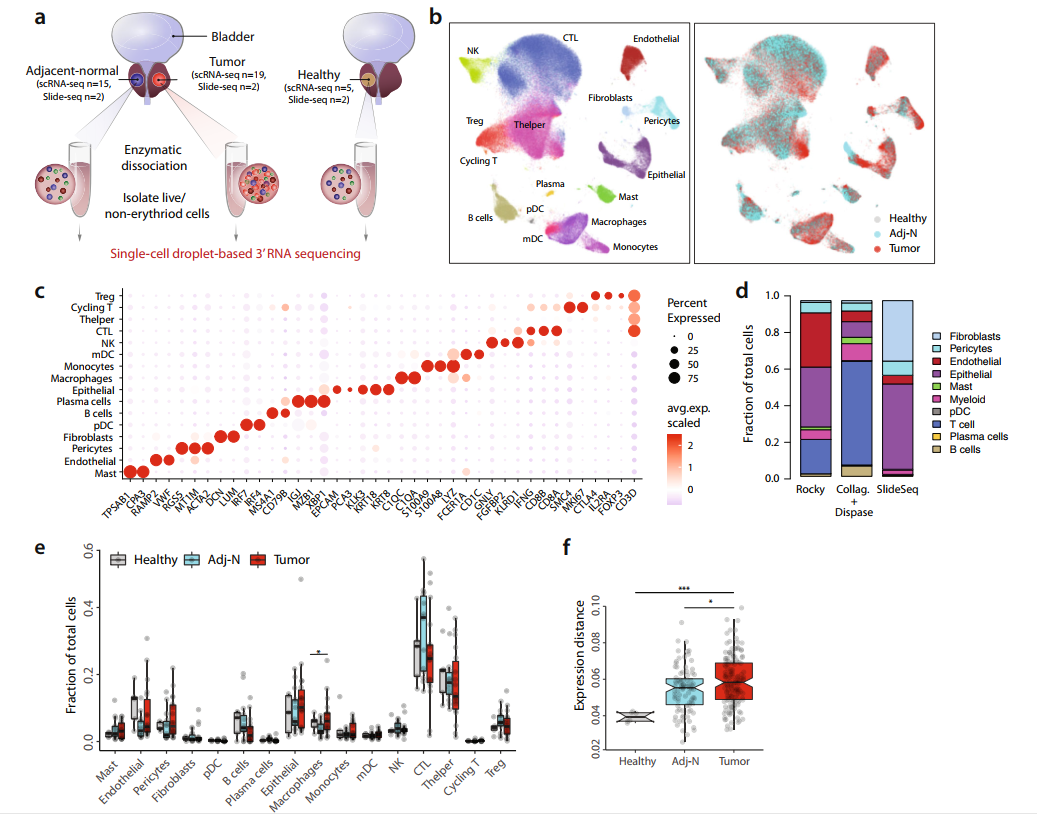
Dissecting the immune suppressive human prostate tumor microenvironment via integrated single-cell and spatial transcriptomic analyses
通过整合单细胞和空间转录组学分析解析人类前列腺肿瘤微环境的免疫抑制特征
Nat Commun. 2023 Feb 7;14(1):663.
doi: 10.1038/s41467-023-36325-2.
PubMed: https://pubmed.ncbi.nlm.nih.gov/36750562/
Abstract
The treatment of low-risk primary prostate cancer entails active surveillance only, while high-risk disease requires multimodal treatment including surgery, radiation therapy, and hormonal therapy. Recurrence and development of metastatic disease remains a clinical problem, without a clear understanding of what drives immune escape and tumor progression. Here, we comprehensively describe the tumor microenvironment of localized prostate cancer in comparison with adjacent normal samples and healthy controls. Single-cell RNA sequencing and high-resolution spatial transcriptomic analyses reveal tumor context dependent changes in gene expression. Our data indicate that an immune suppressive tumor microenvironment associates with suppressive myeloid populations and exhausted T-cells, in addition to high stromal angiogenic activity. We infer cell-to-cell relationships from high throughput ligand-receptor interaction measurements within undissociated tissue sections. Our work thus provides a highly detailed and comprehensive resource of the prostate tumor microenvironment as well as tumor-stromal cell interactions.
低危原发性前列腺癌的治疗仅需要积极监测,而高危前列腺癌需要包括手术、放疗和激素治疗在内的多模式治疗。转移性疾病的复发和发展仍然是一个临床难题,目前尚不清楚是什么驱动了免疫逃逸和肿瘤进展。在这里,我们全面描述了局限性前列腺癌的肿瘤微环境,并与癌旁正常样本和健康对照进行了比较。单细胞RNA测序和高分辨率空间转录组分析揭示了肿瘤环境依赖性的基因表达变化。我们的数据表明,除高基质血管生成活性外,免疫抑制性肿瘤微环境还与抑制性髓系细胞群和耗竭的T细胞相关。我们通过未解离组织切片内的高通量配体-受体相互作用检测来推断细胞-细胞关系。综上,我们的工作提供了前列腺肿瘤微环境以及肿瘤-间质细胞相互作用的高度详细和全面的资源。
07
Cancer Discov. 20230206
✦
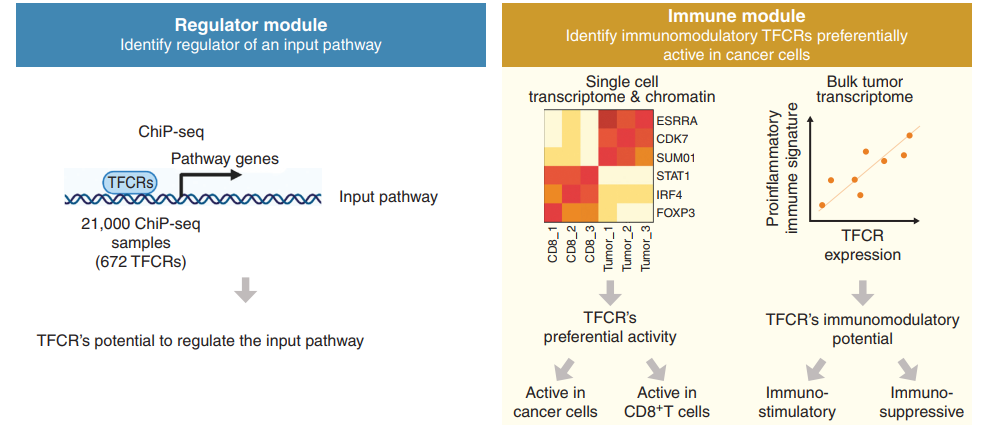
Discovery of Targets for Immune-Metabolic Antitumor Drugs Identifies Estrogen-Related Receptor Alpha
免疫代谢抗肿瘤药物靶点的发现-以雌激素相关受体α为例
Cancer Discov. 2023 Feb 6;OF1-OF30.
doi: 10.1158/2159-8290.CD-22-0244.
PubMed: https://pubmed.ncbi.nlm.nih.gov/36745048/
Abstract
Drugs that kill tumors through multiple mechanisms have the potential for broad clinical benefits. Here, we first developed an in silico multiomics approach (BipotentR) to find cancer cell-specific regulators that simultaneously modulate tumor immunity and another oncogenic pathway and then used it to identify 38 candidate immune-metabolic regulators. We show the tumor activities of these regulators stratify patients with melanoma by their response to anti-PD-1 using machine learning and deep neural approaches, which improve the predictive power of current biomarkers. The topmost identified regulator, ESRRA, is activated in immunotherapy-resistant tumors. Its inhibition killed tumors by suppressing energy metabolism and activating two immune mechanisms: (i) cytokine induction, causing proinflammatory macrophage polarization, and (ii) antigen-presentation stimulation, recruiting CD8+ T cells into tumors. We also demonstrate a wide utility of BipotentR by applying it to angiogenesis and growth suppressor evasion pathways. BipotentR (http://bipotentr.dfci.harvard.edu/) provides a resource for evaluating patient response and discovering drug targets that act simultaneously through multiple mechanisms.
通过多种机制杀伤肿瘤的药物具有广泛的临床获益潜力。在本研究中,我们首先开发了一种计算机模拟多组学方法(BipotentR),以寻找同时调节肿瘤免疫和另一种致癌通路的癌症细胞特异性调节因子,然后使用该方法确定了38个候选免疫代谢调节因子。我们展示了这些调节因子的肿瘤活性,这些调节因子使用机器学习和深度神经方法,根据患者对抗PD -1的反应对黑色素瘤患者进行分层,这两种方法提高了当前生物标志物的预测能力。在免疫治疗耐药的肿瘤中,ESRRA被激活。其抑制作用通过抑制能量代谢和激活两种免疫机制杀死肿瘤:(i)细胞因子诱导,引起促炎巨噬细胞极化;(ii)抗原提呈刺激,募集CD8+ T细胞进入肿瘤。我们还通过将BipotentR应用于血管生成和生长抑制逃逸途径,证明了它的广泛用途。BipotentR (http://bipotentr.dfci.harvard.edu/)为评估患者应答情况和发现通过多种机制同时起作用的药物靶点提供了资源。
08
Mol Cancer. 20230204
✦
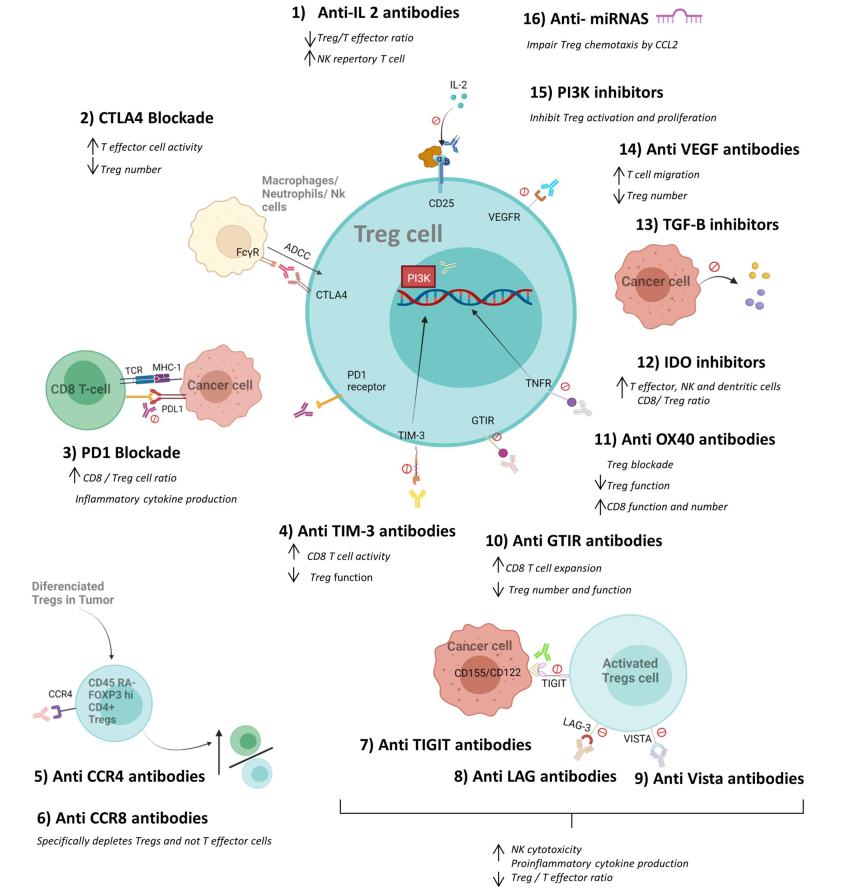
Regulatory cells and the effect of cancer immunotherapy
调节性细胞对癌症免疫治疗的影响
Mol Cancer. 2023 Feb 4;22(1):26.
doi: 10.1186/s12943-023-01714-0.
PubMed: https://pubmed.ncbi.nlm.nih.gov/36739406/
Abstract
Several mechanisms and cell types are involved in the regulation of the immune response. These include mostly regulatory T cells (Tregs), regulatory macrophages (Mregs), myeloid suppressor cells (MDSCs) and other regulatory cell types such as tolerogenic dendritic cells (tolDCs), regulatory B cells (Bregs), and mesenchymal stem cells (MSCs). These regulatory cells, known for their ability to suppress immune responses, can also suppress the anti-tumor immune response. The infiltration of many regulatory cells into tumor tissues is therefore associated with a poor prognosis. There is growing evidence that elimination of Tregs enhances anti-tumor immune responses. However, the systemic depletion of Treg cells can simultaneously cause deleterious autoimmunity. Furthermore, since regulatory cells are characterized by their high level of expression of immune checkpoints, it is also expected that immune checkpoint inhibitors perform part of their function by blocking these molecules and enhancing the immune response. This indicates that immunotherapy does not only act by activating specific effector T cells but can also directly or indirectly attenuate the suppressive activity of regulatory cells in tumor tissues. This review aims to draw together our current knowledge about the effect of immunotherapy on the various types of regulatory cells, and how these effects may be beneficial in the response to immunotherapy.
多种机制和细胞类型参与免疫应答的调节。这些细胞主要包括调节性T细胞(Tregs)、调节性巨噬细胞(Mregs)、髓样抑制细胞(MDSCs)和其他调节性细胞类型,如耐受性树突状细胞(tolDCs)、调节性B细胞(Bregs)和间充质干细胞(MSCs)。这些调节细胞,以其抑制免疫反应的能力而闻名,也可以抑制抗肿瘤免疫反应。因此,许多调节性细胞在肿瘤组织中的浸润与不良预后相关。越来越多的证据表明,清除Tregs可增强抗肿瘤免疫应答。然而,全身性清除Treg细胞可同时引起有害的自身免疫。此外,由于调节性细胞的特点是其高水平表达免疫检查点,因此预期免疫检查点抑制剂也可以通过阻断这些分子和增强免疫反应来发挥部分功能。这表明免疫治疗不仅通过激活特异性效应T细胞发挥作用,而且可以直接或间接减弱肿瘤组织中调节性T细胞的抑制活性。这篇综述旨在总结我们目前关于免疫治疗对各种类型调节性细胞的影响的知识,以及如何使这些影响有利于对免疫治疗的应答。
Keywords:
Checkpoint inhibitors; Immunotherapy; MDSCs; Regulatory cells; TAMs; Tregs; Tumor.
检查点抑制剂;免疫治疗;髓源性抑制细胞;调节细胞;肿瘤相关巨噬细胞;亚群;肿瘤。
09
Nat Commun. 20230202
✦
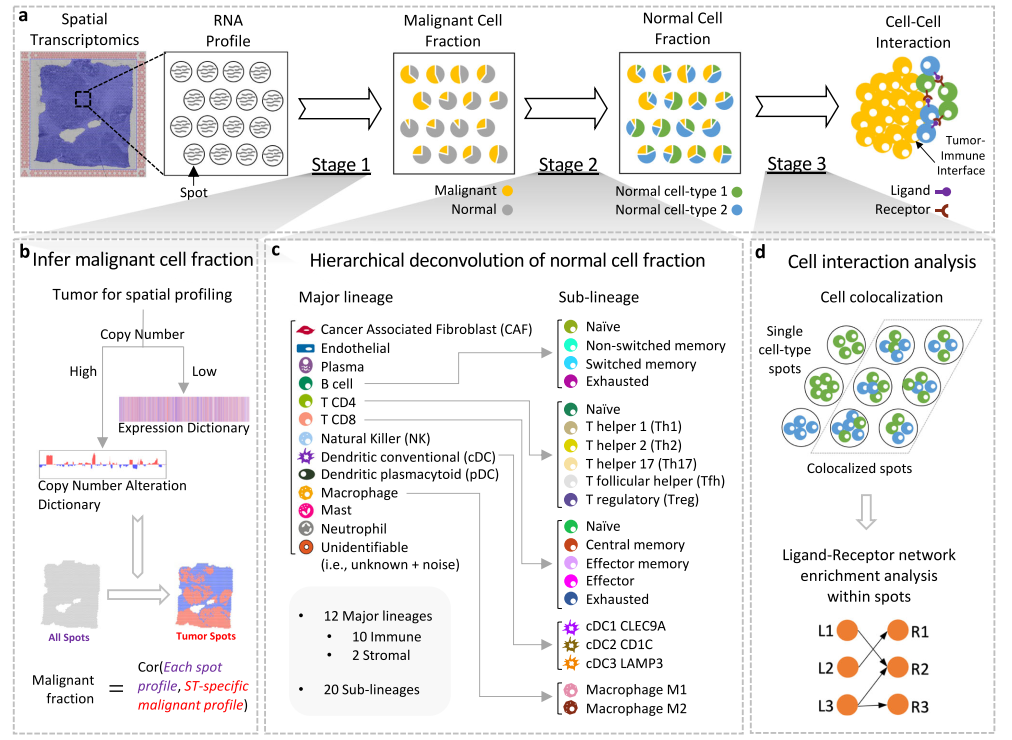
Estimation of cell lineages in tumors from spatial transcriptomics data
从空间转录组学数据推断肿瘤中的细胞谱系
Nat Commun. 2023 Feb 2;14(1):568.
doi: 10.1038/s41467-023-36062-6.
PubMed: https://pubmed.ncbi.nlm.nih.gov/36732531/
Abstract
Spatial transcriptomics (ST) technology through in situ capturing has enabled topographical gene expression profiling of tumor tissues. However, each capturing spot may contain diverse immune and malignant cells, with different cell densities across tissue regions. Cell type deconvolution in tumor ST data remains challenging for existing methods designed to decompose general ST or bulk tumor data. We develop the Spatial Cellular Estimator for Tumors (SpaCET) to infer cell identities from tumor ST data. SpaCET first estimates cancer cell abundance by integrating a gene pattern dictionary of copy number alterations and expression changes in common malignancies. A constrained regression model then calibrates local cell densities and determines immune and stromal cell lineage fractions. SpaCET provides higher accuracy than existing methods based on simulation and real ST data with matched double-blind histopathology annotations as ground truth. Further, coupling cell fractions with ligand-receptor coexpression analysis, SpaCET reveals how intercellular interactions at the tumor-immune interface promote cancer progression.
空间转录组学(ST)技术通过原位捕获实现了肿瘤组织的基因表达谱分析。然而,每个捕获点可能包含不同的免疫和恶性细胞,不同组织区域的细胞密度不同。对于现有的用于分解一般ST或bulk肿瘤数据的方法来说,肿瘤ST数据中的细胞类型反卷积仍然具有挑战性。我们开发了肿瘤空间细胞推断器(SpaCET)来从肿瘤ST数据中推断细胞类型。SpaCET首先通过整合常见恶性肿瘤中拷贝数改变和表达变化的基因模式来评估癌细胞丰度。然后,通过一个约束回归模型校准局部细胞密度,并确定免疫细胞和基质细胞的谱系组分。与现有的基于模拟和真实ST数据、匹配的双盲组织病理学注释的方法相比,SpaCET具有更高的准确性。此外,通过将细胞组分与配体-受体共表达分析耦联,SpaCET揭示了肿瘤-免疫的细胞间相互作用是如何促进癌症进展的。
10
Nature. 20230201-1
✦
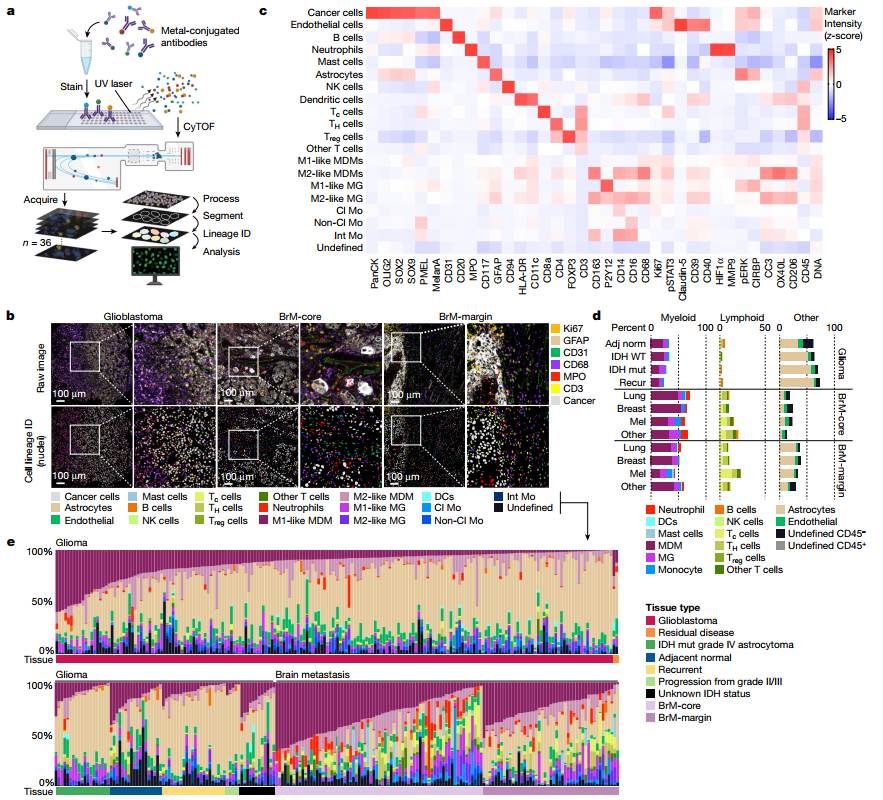
Single-cell spatial immune landscapes of primary and metastatic brain tumours
原发性和转移性脑肿瘤的单细胞空间免疫景观
Nature. 2023 Feb 1.
doi: 10.1038/s41586-022-05680-3.
PubMed: https://pubmed.ncbi.nlm.nih.gov/36725935/
Abstract
Single-cell technologies have enabled the characterization of the tumour microenvironment at unprecedented depth and have revealed vast cellular diversity among tumour cells and their niche. Anti-tumour immunity relies on cell-cell relationships within the tumour microenvironment1,2, yet many single-cell studies lack spatial context and rely on dissociated tissues3. Here we applied imaging mass cytometry to characterize the immunological landscape of 139 high-grade glioma and 46 brain metastasis tumours from patients. Single-cell analysis of more than 1.1 million cells across 389 high-dimensional histopathology images enabled the spatial resolution of immune lineages and activation states, revealing differences in immune landscapes between primary tumours and brain metastases from diverse solid cancers. These analyses revealed cellular neighbourhoods associated with survival in patients with glioblastoma, which we leveraged to identify a unique population of myeloperoxidase (MPO)-positive macrophages associated with long-term survival. Our findings provide insight into the biology of primary and metastatic brain tumours, reinforcing the value of integrating spatial resolution to single-cell datasets to dissect the microenvironmental contexture of cancer.
单细胞技术使肿瘤微环境的表征达到了前所未有的深度,并揭示了肿瘤细胞及其周围环境的巨大细胞多样性。抗肿瘤免疫依赖于肿瘤微环境中的细胞-细胞间关系,但许多单细胞研究缺乏空间背景,而依赖于分离的组织。在这里,我们应用成像质谱细胞术来表征139例高级别胶质瘤和46例脑转移瘤患者的免疫特征。对389张高维组织病理学图像中的超过110万个细胞进行的单细胞分析使免疫谱系和激活状态的空间分辨率成为可能,揭示了不同实体癌原发肿瘤和脑转移瘤之间的免疫景观差异。这些分析揭示了与胶质母细胞瘤患者生存相关的细胞相邻关系,我们利用这一分析确定了与长期生存相关的髓过氧化物酶(MPO)阳性巨噬细胞群体。我们的发现为原发性和转移性脑肿瘤的生物学提供了见解,强化了将空间分辨率整合到单细胞数据集以解析癌症微环境背景的价值。
11
Nature. 20230201-1
✦
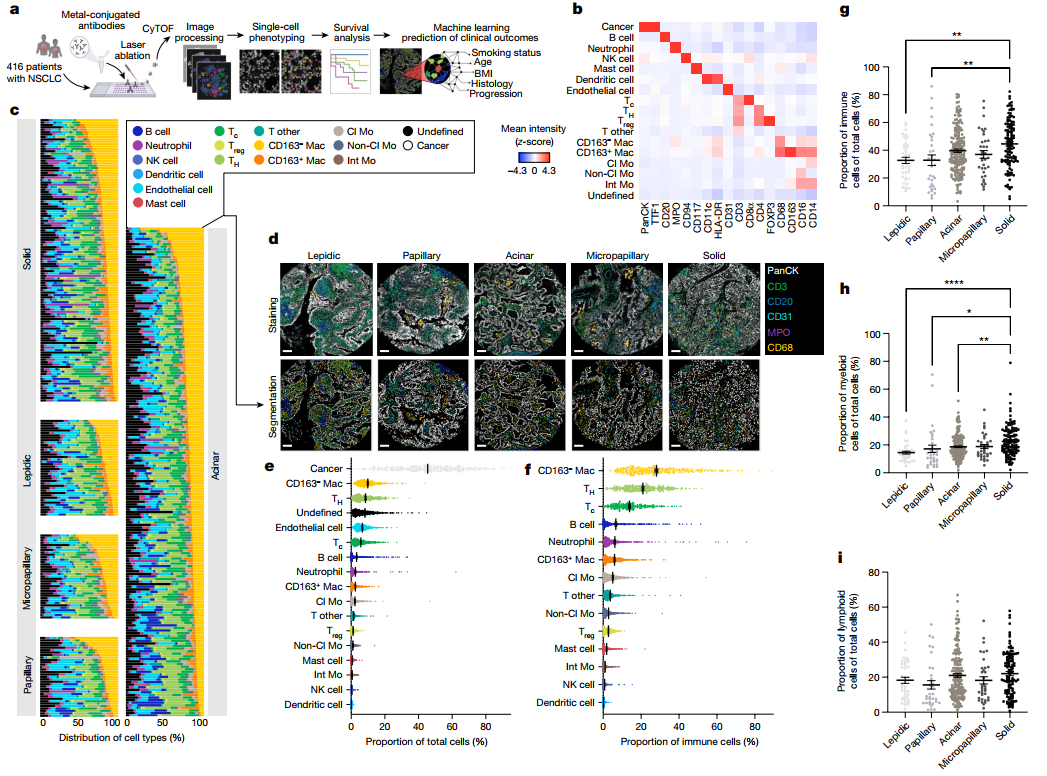
Single-cell spatial landscapes of the lung tumour immune microenvironment
肺癌免疫微环境的单细胞空间景观
Nature. 2023 Feb 1.
doi: 10.1038/s41586-022-05672-3.
PubMed: https://pubmed.ncbi.nlm.nih.gov/36725934/
Abstract
Single-cell technologies have revealed the complexity of the tumour immune microenvironment with unparalleled resolution. Most clinical strategies rely on histopathological stratification of tumour subtypes, yet the spatial context of single-cell phenotypes within these stratified subgroups is poorly understood. Here we apply imaging mass cytometry to characterize the tumour and immunological landscape of samples from 416 patients with lung adenocarcinoma across five histological patterns. We resolve more than 1.6 million cells, enabling spatial analysis of immune lineages and activation states with distinct clinical correlates, including survival. Using deep learning, we can predict with high accuracy those patients who will progress after surgery using a single 1-mm2 tumour core, which could be informative for clinical management following surgical resection. Our dataset represents a valuable resource for the non-small cell lung cancer research community and exemplifies the utility of spatial resolution within single-cell analyses. This study also highlights how artificial intelligence can improve our understanding of microenvironmental features that underlie cancer progression and may influence future clinical practice.
单细胞技术以无与伦比的分辨率揭示了肿瘤免疫微环境的复杂性。大多数临床策略依赖于肿瘤亚型的组织病理学分层,但对这些分层的亚组中单细胞表型的空间背景了解甚少。在这里,我们应用成像质谱细胞术来表征来自416例肺腺癌患者5种组织学模式样本的肿瘤和免疫景观。我们解析了160多万个细胞,从而能够对具有不同临床相关性(包括生存)的免疫谱系和激活状态进行空间分析。利用深度学习,我们可以使用单个1 mm2的肿瘤组织来预测术后进展的患者,这可以为手术切除后的临床管理提供帮助。我们的数据集代表了非小细胞肺癌研究领域的宝贵资源,并例证了单细胞分析中空间分辨率的效用。本研究还强调了人工智能如何提高我们对癌症进展相关的微环境特征的理解,这很可能会影响未来的临床实践。
12
J Clin Invest. 20230201-1
✦
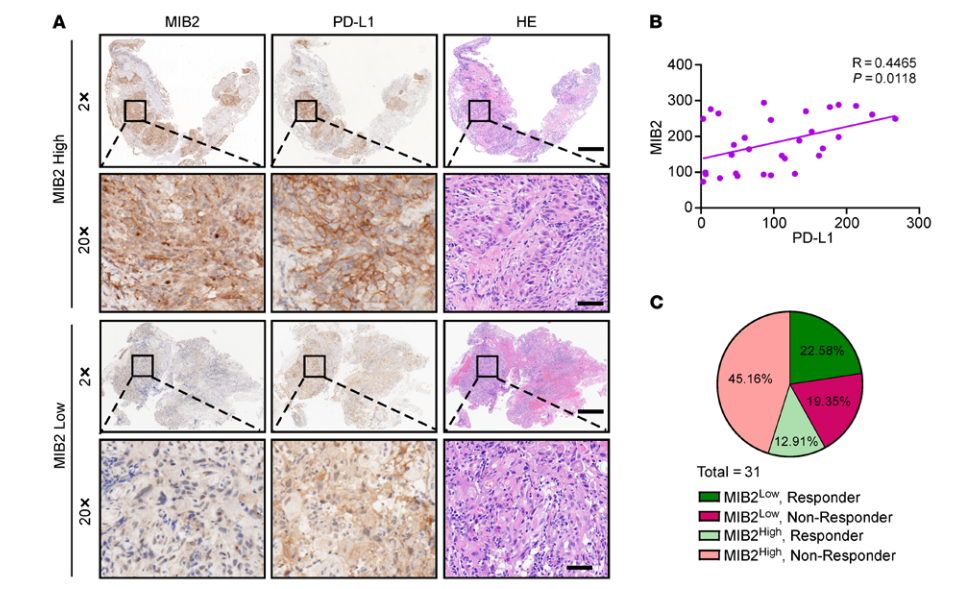
PD-L1 translocation to the plasma membrane enables tumor immune evasion through MIB2 ubiquitination
PD-L1转位至质膜可通过MIB2泛素化实现肿瘤免疫逃逸
J Clin Invest. 2023 Feb 1;133(3):e160456.
doi: 10.1172/JCI160456.
PubMed: https://pubmed.ncbi.nlm.nih.gov/36719382/
Abstract
Programmed death-ligand 1 (PD-L1), a critical immune checkpoint ligand, is a transmembrane protein synthesized in the endoplasmic reticulum of tumor cells and transported to the plasma membrane to interact with programmed death 1 (PD-1) expressed on T cell surface. This interaction delivers coinhibitory signals to T cells, thereby suppressing their function and allowing evasion of antitumor immunity. Most companion or complementary diagnostic devices for assessing PD-L1 expression levels in tumor cells used in the clinic or in clinical trials require membranous staining. However, the mechanism driving PD-L1 translocation to the plasma membrane after de novo synthesis is poorly understood. Herein, we showed that mind bomb homolog 2 (MIB2) is required for PD-L1 transportation from the trans-Golgi network (TGN) to the plasma membrane of cancer cells. MIB2 deficiency led to fewer PD-L1 proteins on the tumor cell surface and promoted antitumor immunity in mice. Mechanistically, MIB2 catalyzed nonproteolytic K63-linked ubiquitination of PD-L1, facilitating PD-L1 trafficking through Ras-associated binding 8-mediated (RAB8-mediated) exocytosis from the TGN to the plasma membrane, where it bound PD-1 extrinsically to prevent tumor cell killing by T cells. Our findings demonstrate that nonproteolytic ubiquitination of PD-L1 by MIB2 is required for its transportation to the plasma membrane and tumor cell immune evasion.
程序性死亡受体配体1 (PD-L1)是一种重要的免疫检查点配体,在肿瘤细胞内质网合成并转运至质膜,与T细胞表面表达的程序性死亡受体1 (PD-1)相互作用。这种相互作用向T细胞传递共抑制信号,从而抑制其功能,逃避抗肿瘤免疫。临床上或临床试验中用于评估肿瘤细胞PD-L1表达水平的大多数伴随或补充诊断需要膜染色。然而,驱动PD-L1合成后转位到质膜的机制尚不清楚。在本研究中,我们发现MIB2是PD-L1从反式高尔基网络(TGN)转运到癌细胞质膜所必需的。MIB2缺陷导致肿瘤细胞表面PD-L1蛋白减少,并促进小鼠抗肿瘤免疫。在机制上,MIB2催化PD-L1的非蛋白水解性泛素化,促进PD-L1通过Rab8介导的胞吐作用从TGN转运至质膜,并在质膜上与PD-1结合,从而阻止T细胞杀伤肿瘤细胞。我们的研究结果表明,MIB2介导的PD-L1的非蛋白水解泛素化是其转运到质膜和引起肿瘤细胞免疫逃逸所必需的。
Keywords:
Cancer; Cancer immunotherapy; Cell Biology; Cellular immune response; Oncology.
癌症;癌症免疫治疗;细胞生物学;细胞免疫反应;肿瘤学。
13
J Clin Invest. 20230201-2
✦
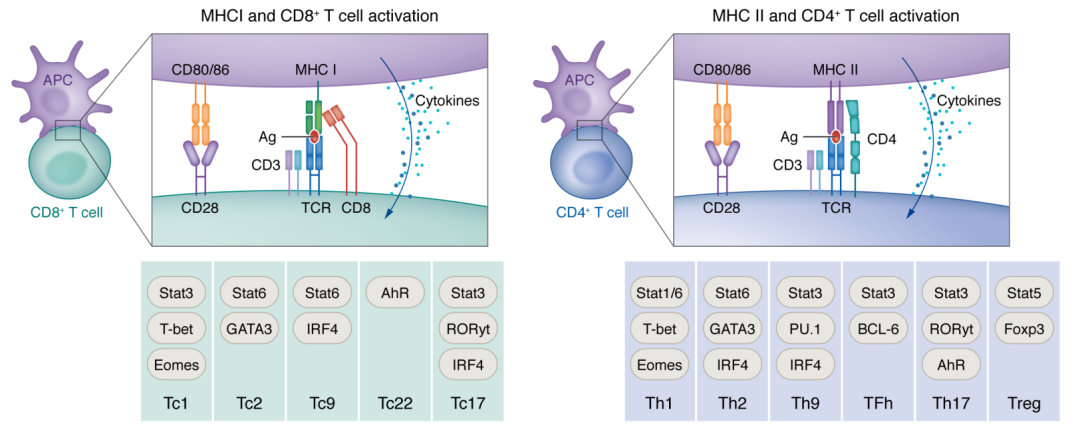
Next-generation antigen-presenting cell immune therapeutics for gliomas
针对胶质瘤的新一代抗原提呈细胞免疫疗法
J Clin Invest. 2023 Feb 1;133(3):e163449.
doi: 10.1172/JCI163449.
PubMed: https://pubmed.ncbi.nlm.nih.gov/36719372/
Abstract
Antigen presentation machinery and professional antigen-presenting cells (APCs) are fundamental for an efficacious immune response against cancers, especially in the context of T cell-centric immunotherapy. Dendritic cells (DCs), the gold standard APCs, play a crucial role in initiating and maintaining a productive antigen-specific adaptive immunity. In recent decades, ex vivo-differentiated DCs from circulating CD14+ monocytes have become the reference for APC-based immunotherapy. DCs loaded with tumor-associated antigens, synthetic peptides, or RNA activate T cells with antitumor properties. This strategy has paved the way for the development of alternative antigen-presenting vaccination strategies, such as monocytes, B cells, and artificial APCs, that have shown effective therapeutic outcomes in preclinical cancer models. The search for alternative APC platforms was initiated by the overall limited clinical impact of DC vaccines, especially in indications such as gliomas, a primary brain tumor known for resistance to any immune intervention. In this Review, we navigate the APC immune therapeutics’ past, present, and future in the context of primary brain tumors.
抗原呈递机制和专职抗原呈递细胞(APCs)是有效抗肿瘤免疫应答的基础,尤其是在以T细胞为中心的免疫治疗中。树突状细胞(DCs)作为”金标准” APC,在启动和维持机体抗原特异性适应性免疫中起着至关重要的作用。近几十年来,从循环CD14+单核细胞体外分化获得DCs已成为基于APC的免疫治疗的标准方法。负载肿瘤相关抗原、合成肽或RNA的DCs可激活具有抗肿瘤特性的T细胞。这一策略为开发其他抗原呈递疫苗策略(如单核细胞、B细胞和人工APC)铺平了道路,这些策略已在临床前肿瘤模型中显示出有效的治疗结局。DC疫苗有限的临床获益,尤其是在胶质瘤(一种对任何免疫干预均有抵抗的原发性脑肿瘤)等适应证中,促使了对替代APC平台的寻找。在这篇综述中,我们在原发性脑肿瘤的背景下探讨了APC免疫疗法的过去、现在和未来。
▉ 强烈推荐
▉ OncoLab实验室网站
本公众号上的往期文章同步发布至对应网站OncoLab实验室。
网站自带检索功能,可以根据关键词进行检索,并且可以根据日期及内容分类进行查看 ,大家可以收藏方便在电脑上查看。
网址是:oncolab.cn
▉ OncoLab学术导航
此外,梳理了一下这几年攒的收藏夹,做了一个导航网页,包含常用网站、文献阅读、试剂订购、基金相关、实用工具、常用数据库等分类内容,并且整合了百度、谷歌、必应三大搜索引擎到检索工具中,欢迎收藏或设置为主页使用~
网址是:dh.oncolab.cn
▉ OncoLab知识星球
OncoLab知识星球也已开通,在学习本公众号内容的过程中如果有什么需要讨论交流的地方可以在星球发表留言,也可以分享一下自己的学习心得体会,其他小伙伴看到了可以积极留言回复,我也会积极参与其中,并时常放一些学习资料在上面,希望大家能够在积极交流互帮互助中共同进步~
该星球用于OncoLab公众号读者交流学习使用,永久免费。

关注本号~

加入读者交流群~
(添加请备注单位姓名)

加入知识星球~
本篇文章来源于微信公众号: OncoLab
 微信扫一扫打赏
微信扫一扫打赏
 支付宝扫一扫打赏
支付宝扫一扫打赏